CW double replacement lab 042706doc rev. This is just one of the solutions for you to be successful.
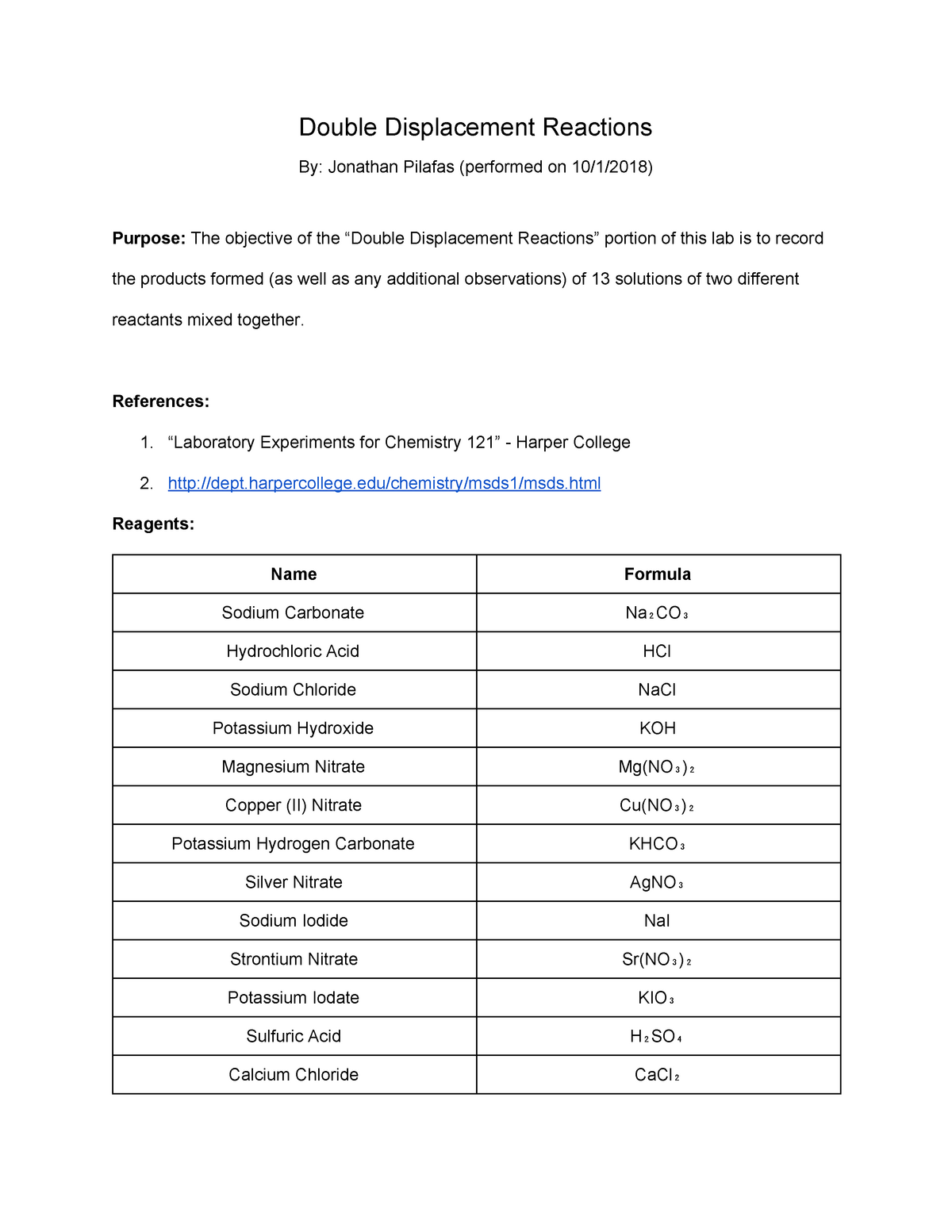
Lab Report Double Displacement Reactions Chm 121 General Chemistry Studocu
For example consider the reaction between aqueous leadII nitrate with aqueous potassium bromide as shown below.
. AX BY AY BX where A and B represent cations and X and Y represent anions. Calcium hydroxide Hydrogen phosphate Æ 4. Read Book Double Replacement Reaction Lab Conclusion AnswersWhen a double replacement reaction occurs the cations and anions switch partners resulting in the formation of water and a new ionic compound or salt which is usually soluble.
June 18th 2018 - Page 7 Double Replacement Reactions and Solubility Post Lab Questions Use a separate sheet of paper to answer the following questions 1 For each combination of an anion and cation testing. It will agreed ease you to see guide chemistry double replacement reactions lab answers as you such as. By searching the title publisher or authors of guide you truly want you can discover them rapidly.
In plastic dropper bottles 01M NaCl twice as many bottles 01MKNO3 01M AgNO3 twice as many bottles 01 M NaI M CuNO32 3 M NaOH twice as many bottles 01 M FeNO33 distilled water gloves. DOUBLE REPLACEMENT REACTIONS PRE-LAB QUESTIONS Name. AB CD AD CB Precipitation Reactions In a precipitation reaction one of the products of the double displacement reaction is a precipitate.
In a precipitation reaction one type of double-replacement reaction two soluble ionic compounds in aqueous solution are mixed and result in an insoluble solid compound called a precipitate. As understood exploit does not recommend that. AX BY AY BX where AB cations and XY anions Eqn.
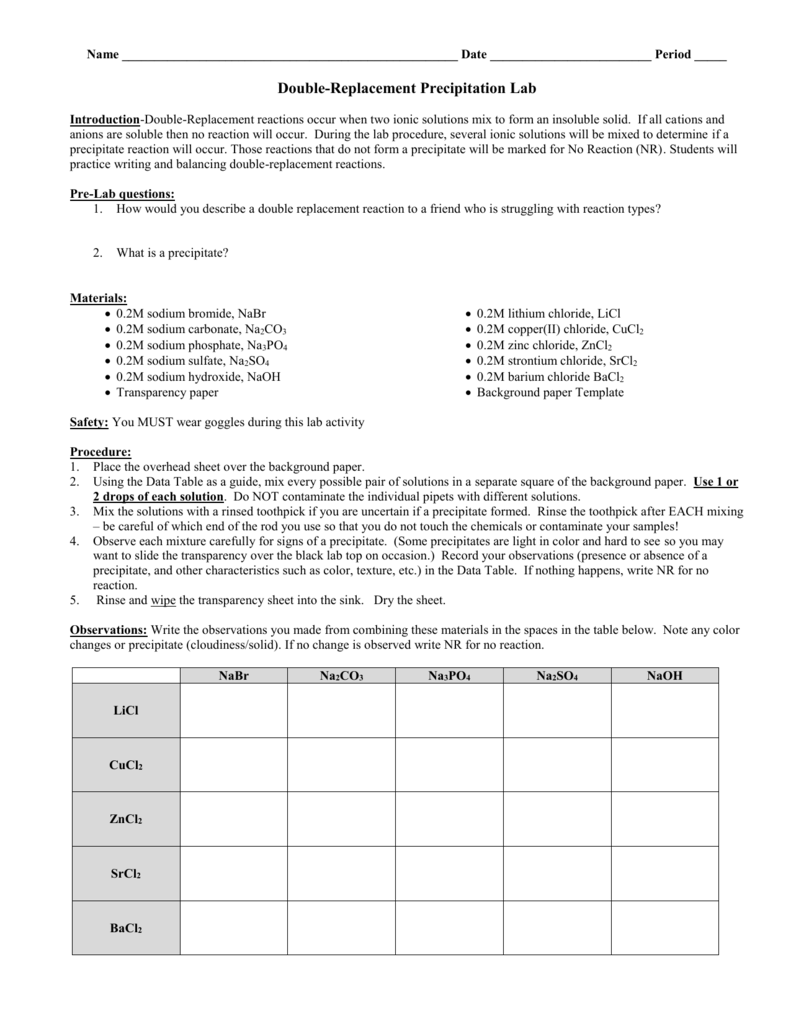
Droplets of reactants such as BaCl 2 and Na 2 SO 4 were dropped into spot plates which created a double replacement reaction. Pre-Lab Discussion Do not copy in your lab book Double-Replacement reactions occur when two ionic solutions mix to form an insoluble solid covalent compound such as water or a gas. Complete the following tables by writing formulas of reactants and observations during the lab.
Purpose of the lab even though the color slightly differed. Ions and form two new ionic compounds. View the full answer.
If you aspire to download and install the. Potassium iodide Lead II Nitrate Æ 7. An example of a general double displacement reaction is shown below where the letters A and C are cations and letters B and D are anions.
Thats a sign that the double-replacement reaction is occurring. In the house workplace or perhaps in your method can be all best area within net connections. This lab helped to enhance knowledge about double-replacement reactions.
Double Replacement Reactions. Here is the double-replacement reaction again but with the follow-up decomposition reaction. A double-replacement reaction can only take place when there are an equal number of cations and anions on the reactant and product side.
When solutions of two dissolved salts are combined and result in the formation of an insoluble salt in the form of a. If all cations and anions are soluble then no reaction will occur. Read PDF Chemistry Double Replacement Reactions Lab Answers Chemistry Double Replacement Reactions Lab Answers Yeah reviewing a books chemistry double replacement reactions lab answers could increase your close contacts listings.
A precipitate is an insoluble solid compound formed during a chemical reaction in solution. Double Replacement Reactions and Solubility Post-Lab Questions. If the substance no longer had an aqueous solution after the double replacement then the substance would be a precipitate.
Q1When two dissolved salts combine to form a precipitate by double decomposition two ions form a precip. 4 rows Reactions that can be classified as double replacements include precipitation reactions. Double Replacement Lab Answers.
Our books collection hosts in multiple countries allowing you to get the most less latency time to download any of our books like this one. Answers double replacement reaction lab conclusion answers is available in our digital library an online access to it is set as public so you can get it instantly. Sodium acetate Calcium sulfide Æ.
5- a From the given table the precipitates formed are Silver Iodide AgI and Copper II phosphate Cu3 PO42 And the salts those are soluble are - N. Hydrogen sulfate Sodium hydrogen carbonate Æ 5. Recognize a double replacement reaction Write the equation for a chemical reaction Required Materials For 100 students 100 mL each of.
A double displacement or metathesis reaction involves two ionic compounds switch partners. The cation of the first molecule exchanges for the anion of the second and the cation of the second for the anion of the first in the general form in Eqn. H Cl aq Na HCO 3aq H 2 CO 3 l NaCl aq H 2 CO 3 CO 2g H 2 O l Since H 2 CO 3 decomposes into CO 2 and H 2 O you can see bubbles forming.
1 A specific example of a double displacement reaction is. Double Replacement Reactions A double replacement reaction is a chemical reactionwhere two reactantioniccompoundsexchange ionsto form two new productcompounds with the same ions. Calcium hydroxide Ammonium chloride Æ 6.
Practice Problem Answers Back to Double Replacement Write correct formulas for the products in these double replacement reactions. Calcium nitride water Æ 3. The products of double replacement reactions will generally be one or more insoluble.
AB CD Æ AD CB 1. Barium chloride Aluminum sulfate Æ 2. Penalty for incorrect answers a.
View the full answer. During the lab procedure several ionic solutions will be mixed to determine if a double replacement reaction will occur. Review you Observations Table.
Complete AND balance each equation below. Neutralization reactions are exothermic and are generally accompanied by a noticeable release of heat. 1 CaOH2 H3PO4--- Ca3PO42 H2O 2 K2CO3 BaCl2--- KCl BaCO3 3 Cd3PO42 NH42S --- CdS NH43PO4 4 CoOH3 HNO3--- CoNO33 H2O 5 AgNO3 KCl --- AgCl KNO3.
All of the solutions studied in this experiment were double-replacement. One of the factors driving a double replacement reaction is the formation of a solid precipitate. The previous lab Lab 8 focused solely on single replacement reactions.
To predict whether a precipitate will form when you mix together two ionic reactants you need to know whether any of the possible products are insoluble. This lab will focus just on double replacement reactions. The chemical equation shows a double replacement reaction.
Compounds were combined together and would generally form a completely different-looking substance. PbNO 3 2 aq KBraq.

Solved Namv Date Section Single E And Double Displacement Chegg Com
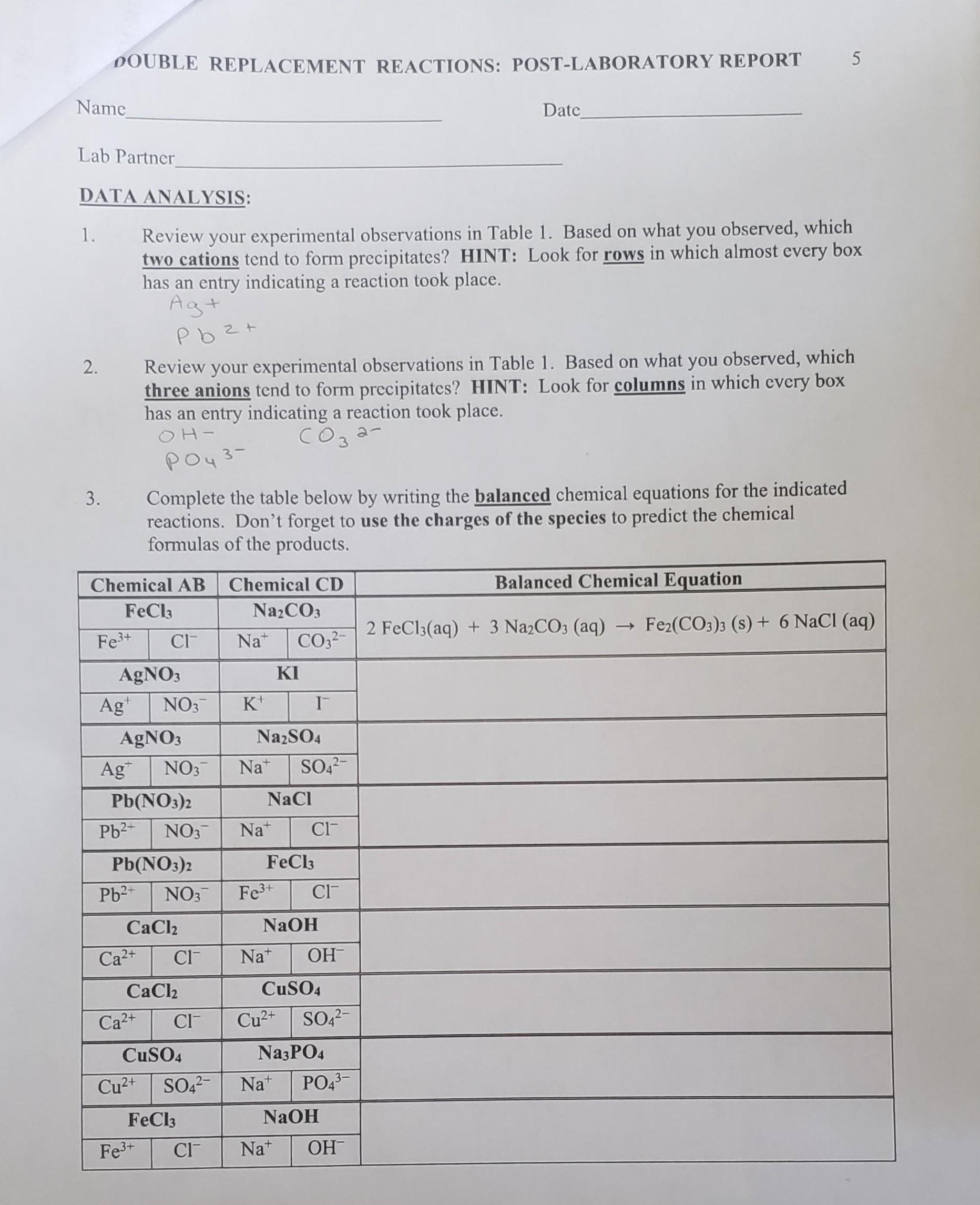
Solved Double Replacement Reactions Post Laboratory Report Chegg Com
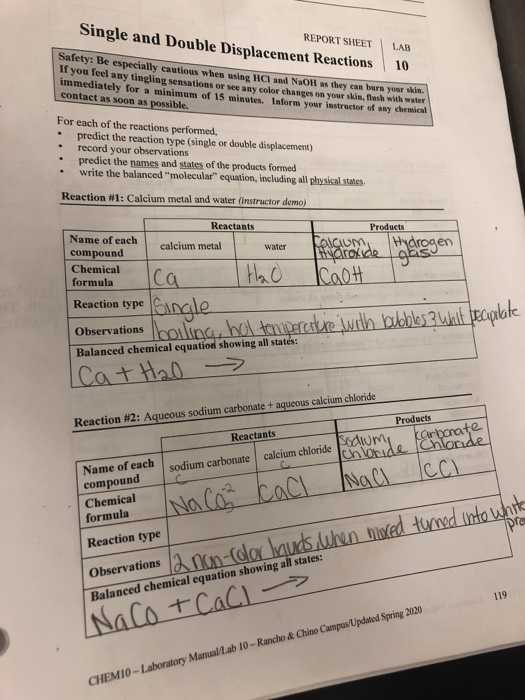
Solved Report Sheet Single And Double Displacement Reactions Chegg Com
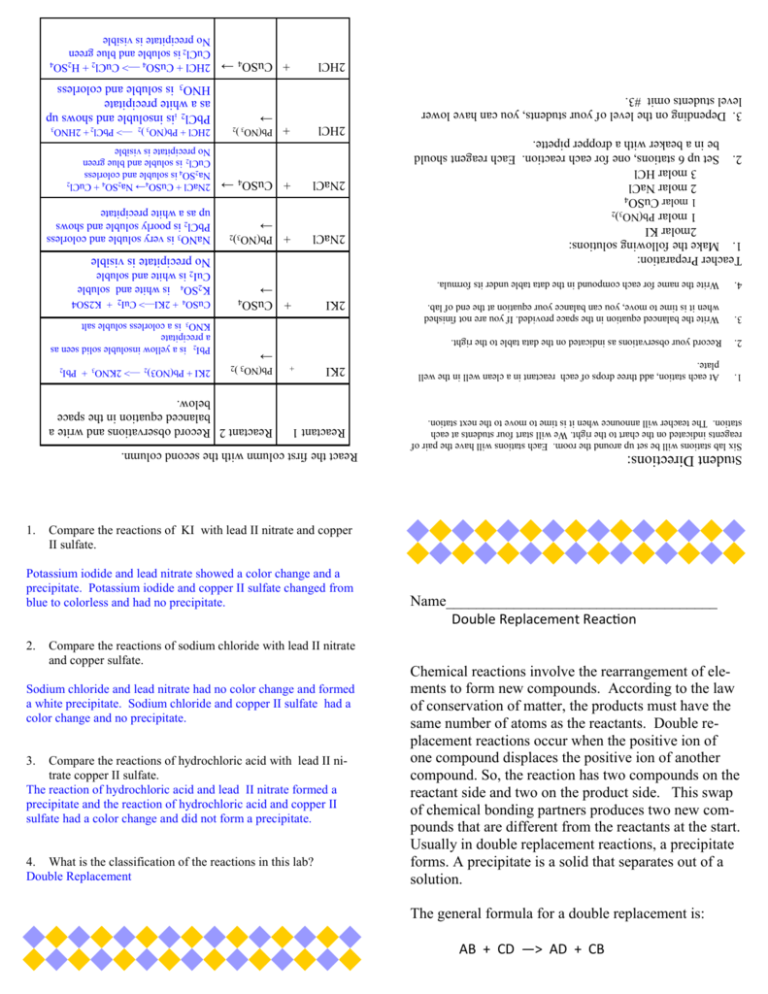
Double Replacement Lab Teacher Answer Key Correct
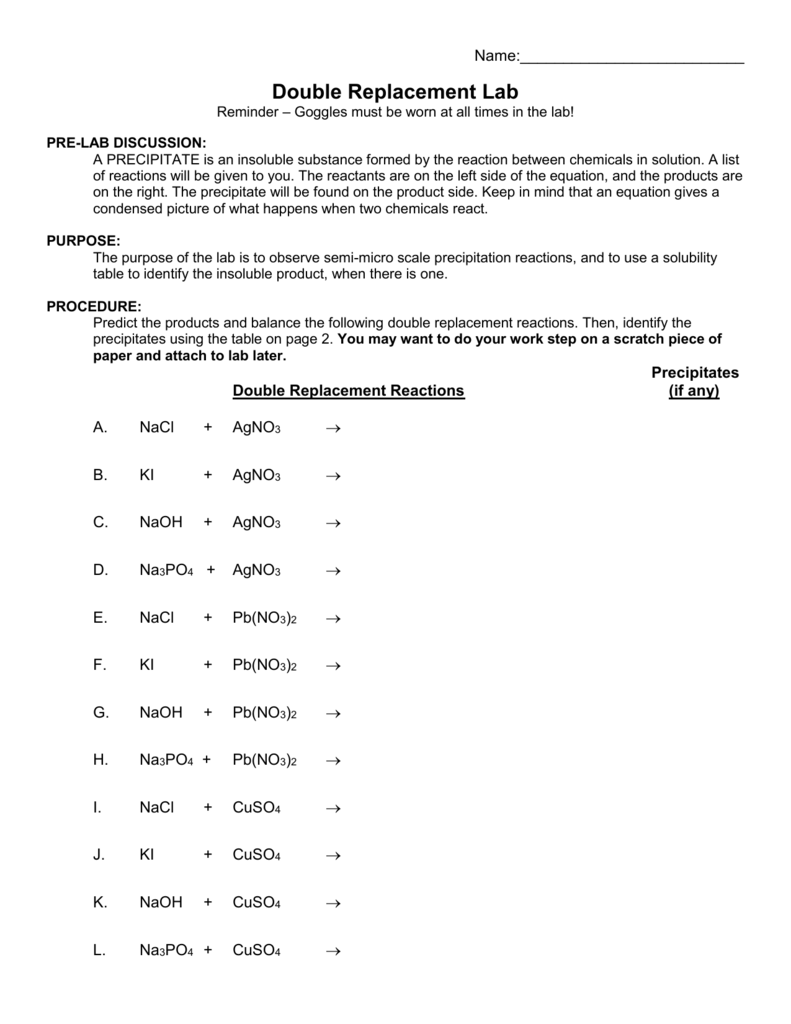
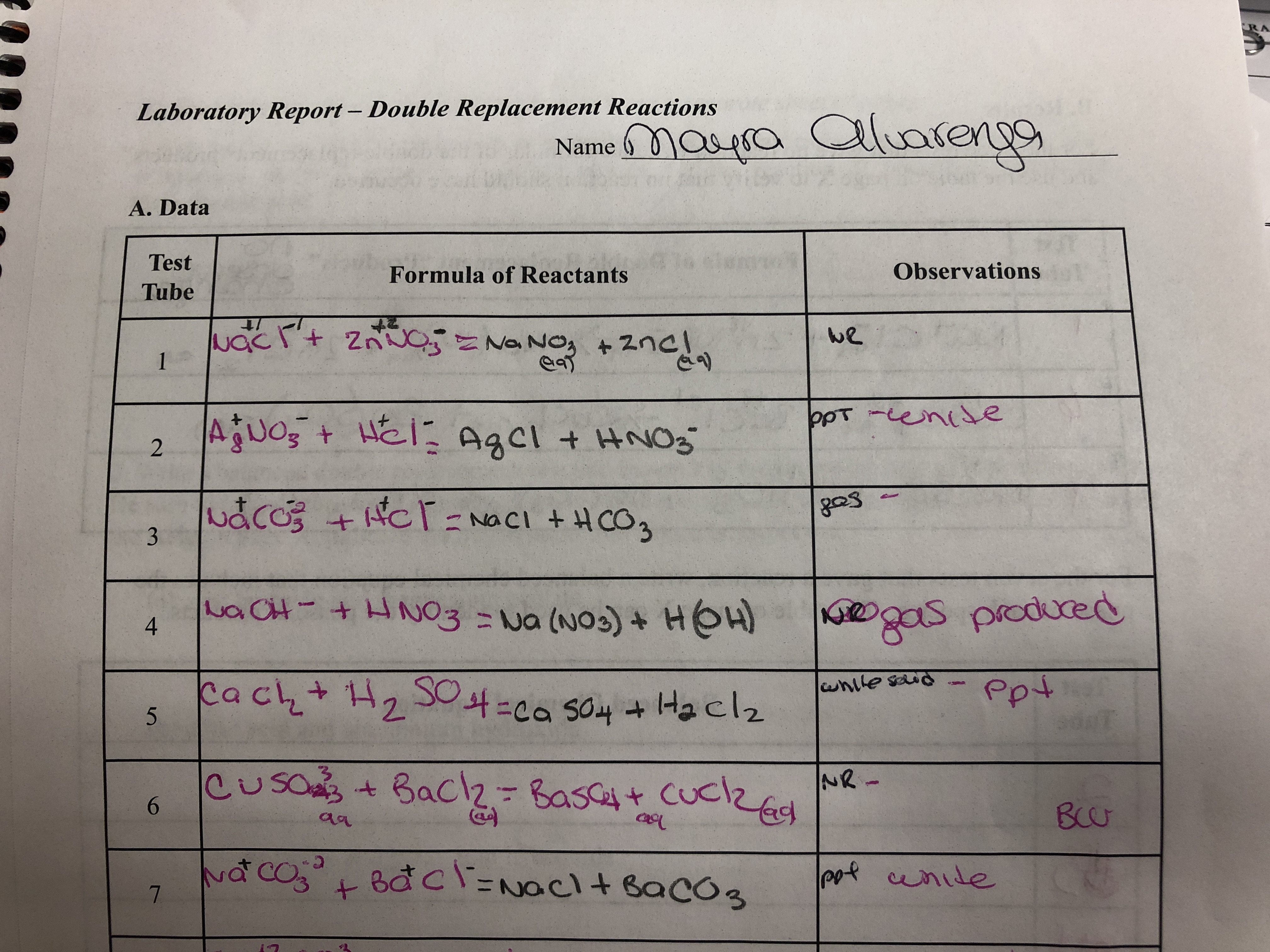
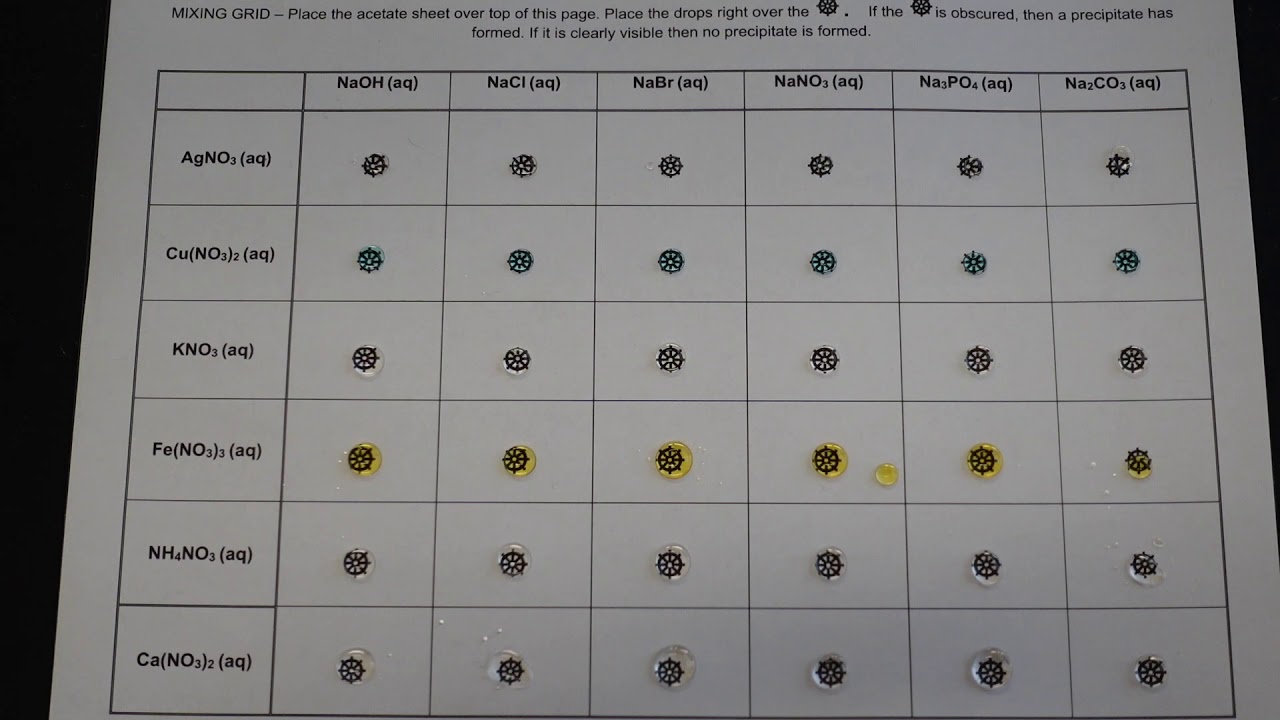
0 comments
Post a Comment